1.1 Clay Minerals:
- The geometrical arrangement of soil particle in a soil mass is known as soil structure.
- The ingredient necessary to give soil deposit cohesion is called clay material.
- Over 50% of soil deposits whose particle diameter is 0.002 mm or less is called clay.
- Clay mineral are composed of tiny crystalline substance of one or more members of a small group of mineral.
- Their particle are very small in size, very flasky in shape and have considrable surface area.
- Thery can only be viewed form electronic microscopic.
- The clay minerals can be divided on the basis of crystalline arrangement into three main group.
1.2 Types of Clay Minerals:
a. Kaoline Mineral:
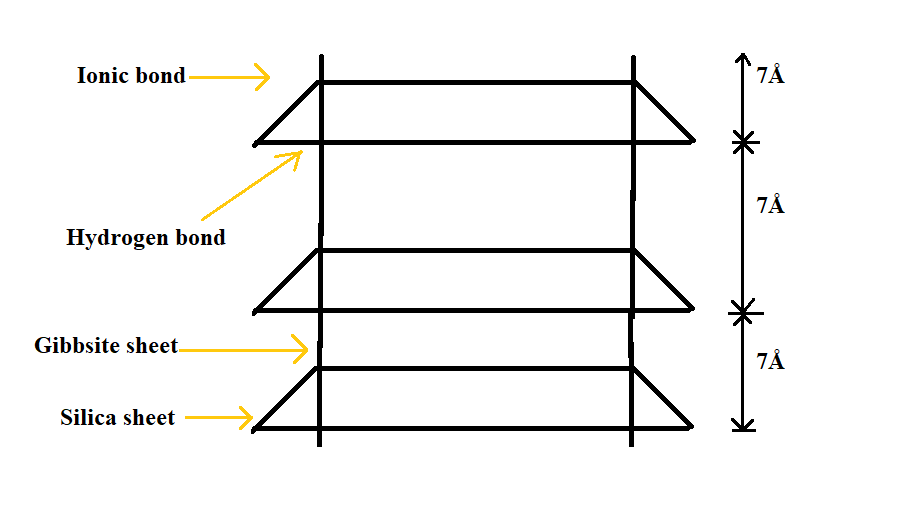
- Kaoline structural unit consists of an alumina sheet combined with a silica sheet.
- The total thickness of structural unit is about 7 Angstrom (7Å).
Where,
1 Å = (10-10m) or (10-7mm)
- Kaoline mineral is formed by stacking one over the other.
- Kaoline mineral are mostly in hexagonal shape.
- The structure unit is joined together by hydrogen bond which is formed between the oxygen of silica sheet and hydroxyls of alumina sheet.
- Most common example of kaoline mineral is china clay.
- Kaoline mineral have thickness is about 0.05 micron.
- The specific surface is 15 m2/gm.
b. Montmorillonite:

- Montmorillonite structural unit consist of alumina sheet sandwiched between two silica sheet.
- The thickness of each structural unit is about 10 Å.
- The two structural unit are joined together by a link between oxygen ions of the two silica sheet. The link is form due to natural attraction of cations in intervening space and due to Vander waal force.
- The negatively charged surface of silica sheet attracts water in the space between two structural unit which causes expansion of material.
- The soil containing large amount of montmorillonite mineral exhibits shrinkage and high swelling characteristics.
- Montmorillonite minerals have thickness of 0.001 micron to 0.005 micron.
- The specific surface is about 800 m2/gm.
c. Illite:
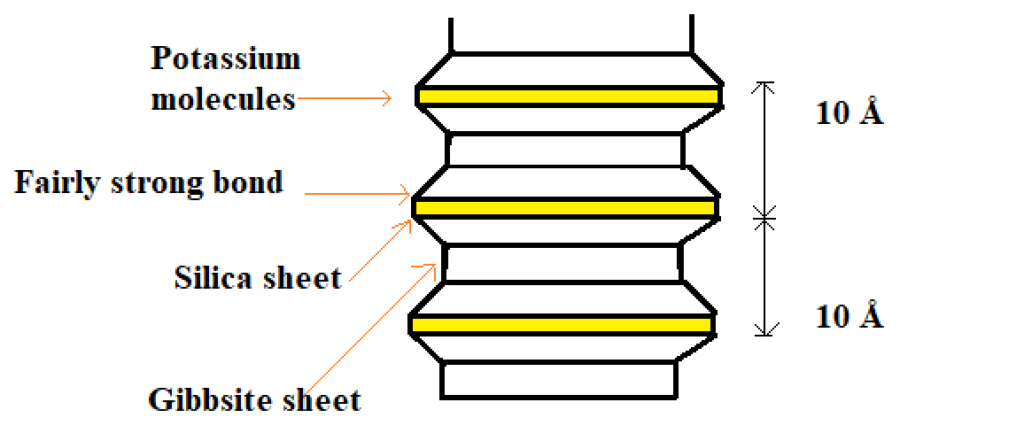
- The structural unit of illite is same as montmorillonite but there is some isomorphous substitution of aluminium for silicon, in the silica sheet and the resultant charge deficiency is balanced by potassium ion which bond the layers in stack.
- Illite crysal does not swell so much in presence of water as montmorillonite.
- The thickness of illite is between 0.005 micron to 0.05 micron.
- The specific surface is about 80 m2/gm.
1.3 Clay Particle Interaction:
- The forces between soil particle is of two types. They are as follows:
- Gravitational force
- Surface force
- Surface for is more dominant over the gravitational force in the case of clay particle which behave like colloids. Colloid is a particle with high specific surface which behavior is influenced by surface energy than mass energy.
- Gravitational force being proportional to mass are important in case of coarse grained soil only.
- Surface for may be attractive or repulsive.
a. Attractive force:

- Vander Waal force
- Hydrogen bond
- Ionic bond
b. Repulsive force:

- Due to similar charge
- Cation repulsion
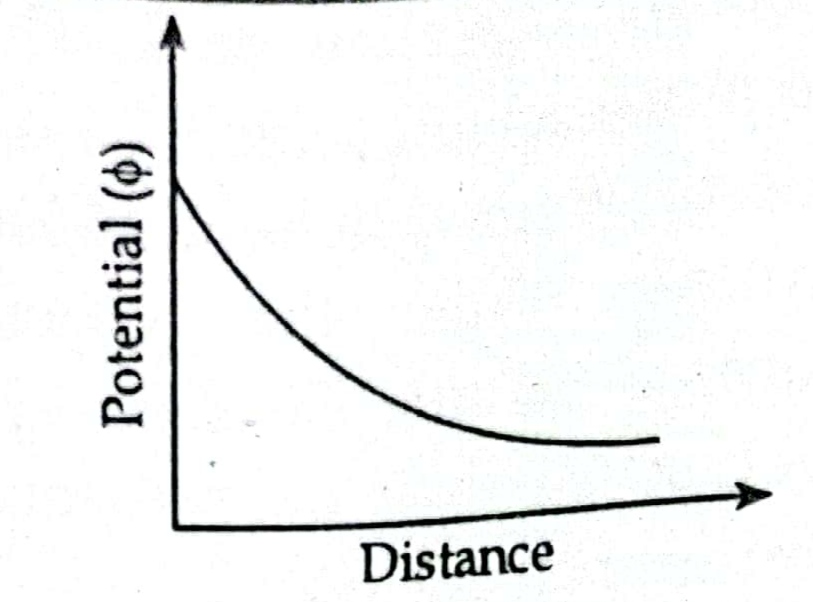
- For a given type of clay in suspension the net force between the adjacent particles at a given distance is the algebraic sum of the repulsive and attractive force acting at the distance.
- Potential force or inter-particle force decreases with increase in distance.
- If total potential energy between two particle decreases the particle will experience attraction and will flocculate but if there is increase in total potential energy the particle will experience repulsion and will disperse. The various factor affecting flocculation or dispersion are electrolyte concentration, temperature, ion valence, pH value, dielectric constant and anion adsorption.
Diffuse Double Layer:
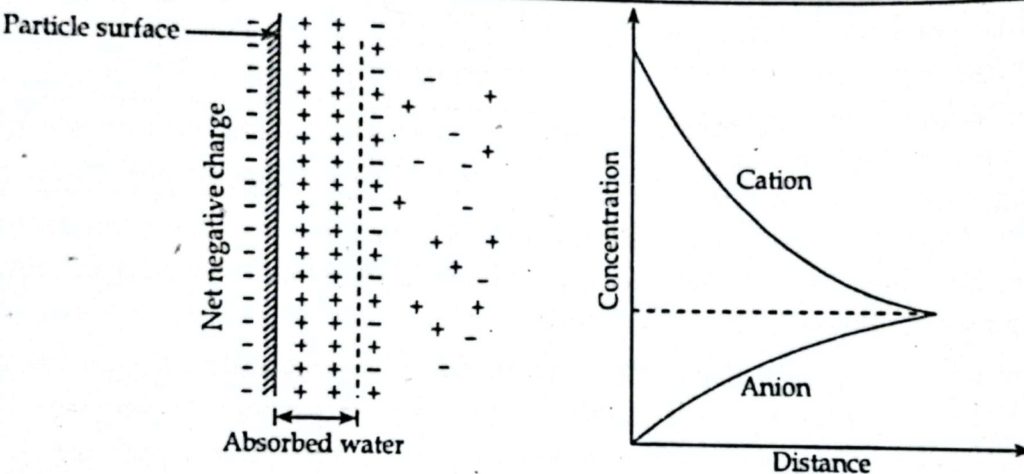
- Normally clay soil are associated with water and its properties are significantly influence with the presence of water.
- Water molecule is dipole and molecules act as a bar magnet and the face of clay mineral carry negative charge except at edge.
- The layer extending from clay particle surface to limit of attraction is known as diffuse double layer.
- The water field in diffuse double layer is known as absorbed water or oriented water.
- Absorbed water imparts plasticity to clay.
1.4 Soil Structure:
The geometric arrangement of soil particle with respect to one another is known as Soil structure. The soil in nature have different strucutre depending upon the particle size and mode of formation.
The various soil mass structure found in natural soil deposit are decribed below:
a. Single – grained structure:
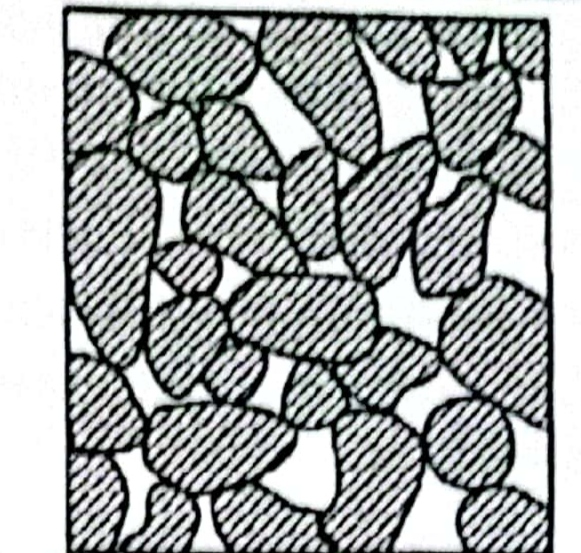
- This kind of structure are found in coarse grained soil.
- Grains which cohesion less and makeup soil like gravel and sand form such kind of structure.
- These grains are large enough and gravitational force is more dominant on them. When these particle deposited they acquire an equilibrium position by each particle being in contact with other surrounding particles.
- Closely the particle are packed together leaving very less void space between them and the structure is denser. When soil particle are loosely packed their is large void space between them and makeup loose soil structure.
b. Flocculated structure:

- Clay particle are very small, flaky in shape and they have large surface area.
- Surface force is more dominant than gravitational force.
- Flocculated structure particle have negative charge on the surface and positive charge on edges. They combine with each other by joining negative surface of particle to positive edge of the other.
- Soil with flocculated structure have large amount of void. So their void ratio is high. These soil are less sensitive to vibration as they formed a strong electrical bond.
c. Honeycomb structure:

- Sometimes smaller soil particle of silt sized when depositing join with one another and form a bridge like structure. They contain large void between those bridge and make the soil very loose in nature. Such type of structure is called honeycomb structure.
- Since honeycomb structure are loose they can support load only static condition. When they are subjected to vibration or shock, the structure collapses and large deformation takes place and soil achieves relatively dense state.
d. Dispersed structure:

- When clay soil are remolded their flocculated structure change. Also, particle change their orientation form edge to face orientation to face to face orientation. Such kind of structure is called dispersed structure.
- Dispersed structure soil have relatively lower volume of void and low void ratio.
- Dispersed structure soil have low shear strength, high compressibility and low permeability.
- When both coarse grained and fine grained particle are present in the soil they make up to two kind of soil structure.
- Coarse grained skeleton
- Cohesive matrix structure
e. Coarse grained skeleton:
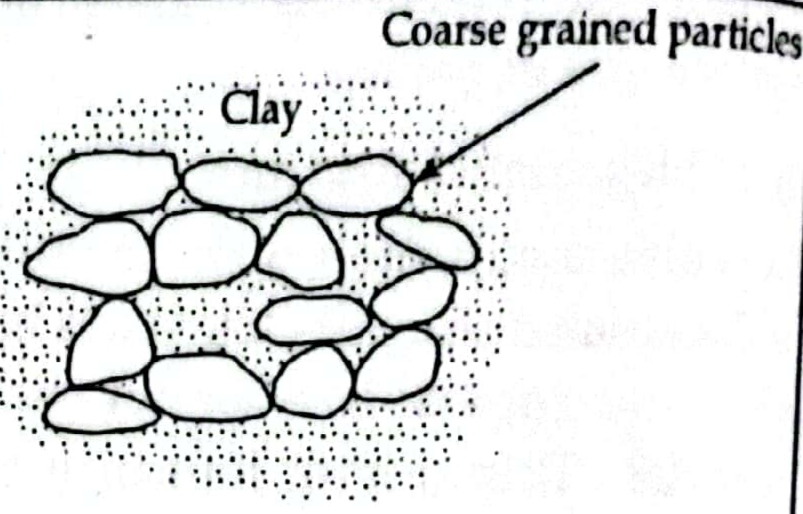
- In coarse grained skeleton coarse grained particle are in large amount than fine grained particle and coarse particle remain in direct contact with other coarse particle forming a framework or a skeleton.
- The space between these large grain is occupied by fine particle.
- In coarse grained skeleton the soil are stable and less compressible and can take heavy load without much deformation.
f. Clay matrix structure or Cohesive matrix structure:
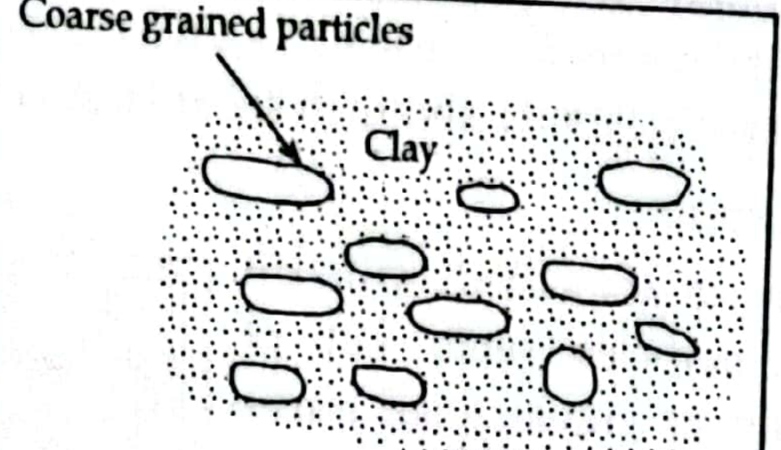
- In clay matrix structure fine grained particle are in large amount than coarse grained particles.
- Coarse grain appear embedded in fine grain and there is no direct particle to particle contact between them.
- Clay matrix structure soil behavior is similar to ordinary clay deposit.
References:
- Terzaghi, Karl, Peck, R.B & John, Wiley (1969) Soil mechanics in engineering practice, New York.
- Arora , K.R (2008), Soil mechanics and foundation engineering, Delhi: Standard Publisher Distribution.

