Fluid Mechanics:
- Fluid mechanics is the branch of science which deals with behavior of fluid (gaseous or liquid).
- Fluid Mechanics Divided into:
| a. Fluid Statics | It is the study of fluid at rest. |
| b. Fluid Kinematics | It is the study of fluid in motion but we don’t consider any forces. |
| c. Fluid Dynamic | It is the study of fluid in motion considering all forces. |
Physical Properties of Liquid
1. Density or Mass Density:
It is defined as the ratio of mass of fluid to the volume of fluid. Its SI unit is kg/m3
Mathematically,
Density (ρ) =(Mass of fluid (m) )/(Volume of fluid (V))
Where,
Density = ρ
Mass of fluid= m
Volume of fluid = V
Note: Density of Water = 1000 kg/ m3
2. Specific Weight or Weight Density
It is defined as the ratio of weight of fluid to the volume of fluid. Its SI unit is N/ or KN/
Mathematically,
Weight Density (γ) = (Weight of fluid(W))/(Volume of fluid(V))
Then,
γ = W/V
Or, γ = (mg)/V
γ = ρ g
Where,
Weight Density or Specific weight= γ
Gravity= g
Density= ρ
Mass= m
Volume = V
3. Specific Volume
It is defined as the ratio of volume per unit mass.
Its SI unit is m3 /kg.
Mathematically,
Specific Volume (Ѵ) = (Volume of fluid(V))/(Mass of fluid(m))
Then,
Ѵ= (V)/m
Ѵ= (1)/(ρ)
4. Specific Gravity or Relative Density
It is defined as the ratio of weight density of liquid to the weight density of standard liquid like water.
Mathematically,
Specific Gravity(S) = (Weight Density of Liquid)/(Weight Density of Standard Liquid)
Also,
Specific Gravity(S) = (Mass Density of Liquid)/(Mass Density of Water)
Note: Specific Gravity of Mercury is 13.6
Viscosity
Viscosity is defined as the property of a fluid which offers resistance to the movement of one layer of fluid over another adjacent layer of the fluid.
Types of Viscosity:
- Dynamic Viscosity
- Kinematic Viscosity
1. Dynamic Viscosity
It is defined as the resistance offered to a layer of fluid when it moves over another layer of fluid.
Its unit is Ns/m2 .
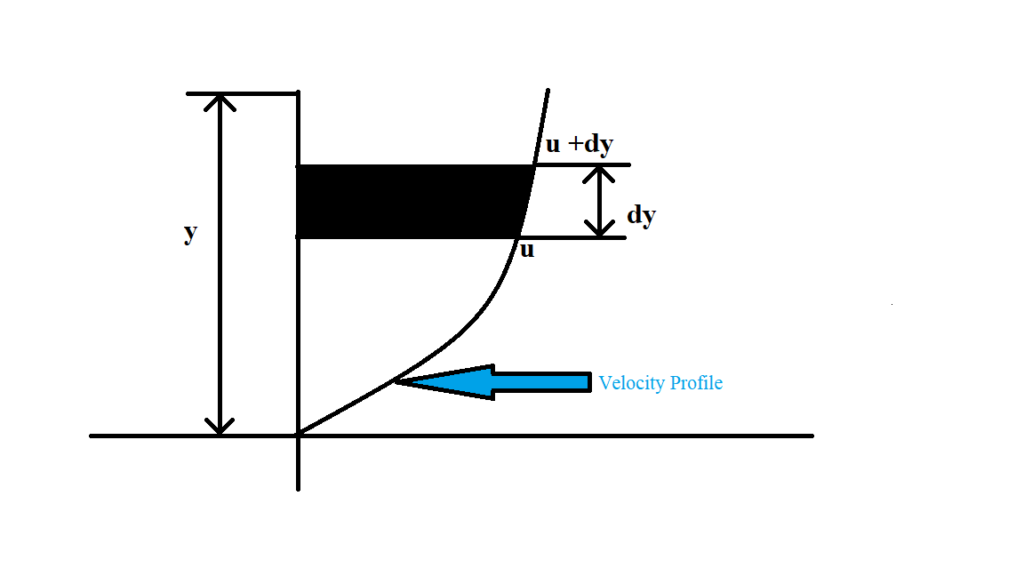
Mathematically,
Rate of shear stress is directly proportional to the velocity gradient.
i.e.
Ʈ∝(du)/(dy)
Ʈ = µ(du)/(dy)
Where,
Dynamic Viscosity = µ
Rate of shear stress = Ʈ
Velocity gradient = du/dy
Then,
µ= (Ʈ)/(du/dy)
Note: 1 Poise = 1/10 Ns/m2
2. Kinematic Viscosity
It is defined as the ratio of dynamic viscosity to density (mass-density) of a fluid. Its unit is m2/s
Mathematically,
Kinematic Viscosity (Ƞ) =(Dynamic Viscosity(µ))/(Density(ρ))
Note: 1 Stoke = 10-4 m2/s
Types of Fluid
Fluid Types
| Ideal Fluid | Ideal Plastic Fluid | Real Fluid | Newtonian Fluid | Non-Newtonian Fluid | Incompressible Fluid | Compressible Fluid |
1. Ideal Fluid
- A fluid which is incompressible and having no viscosity is known as an ideal fluid. It is only an imaginary fluid as all the fluids which exist have some viscosity.
2. Ideal Plastic Fluid
- A fluid which follows Newton’s law of viscosity.
- Example: Toothpaste can be considered ideal plastic fluid.
3. Real Fluid
- A fluid which possesses viscosity is known as real fluid.
- All the fluids in actual practice are real fluid.
- Example: Water, Air etc.
4. Newtonian Fluid
- A real fluid in which shear stress is directly proportional to the rate of shear strain or velocity gradient is known as Newtonian fluid.
- Example: Water, Kerosene, Benzene etc.
5. Non-Newtonian Fluid
- A real fluid in which shear stress is not directly proportional to the rate of shear strain or velocity gradient is known as a Non-Newtonian fluid.
- Example: Ink, Blood, Honey etc.
6. Incompressible Fluid
- A fluid in which the density of fluid does not change with change in external force or pressure is known as incompressible fluid. All liquid are considered in this category.
7. Compressible Fluid
- A fluid in which the density of fluid changes with change in external force or pressure is known as compressible fluid. All gases are considered in this category.
Graphical representation of different fluids:

Tabular representation of fluid types:
| Types of fluid | Density | Viscosity |
| Ideal Fluid | Constant | Zero |
| Real Fluid | Variable | Non-Zero |
| Newtonian Fluid | Constant/Variable | µ=(Ʈ)/(du/dy) |
| Non-Newtonian Fluid | Constant/Variable | µ (Ʈ)/(du/dy) |
| Incompressible Fluid | Constant | Non-zero/Zero |
| Compressible Fluid | Variable | Non-zero/Zero |
Concept of continuum in fluid mechanics
If kn=λ /L is less than 0.01 then it is continuum.
A continuous and homogenous fluid media is known as continuum. Analysis of fluid flow problems is made by a concept that treats fluid as continuous media. All cavities (voids) between the molecules are neglected.
The physical properties like density, temperature, velocity, pressure etc. are the define as continuous function of space and time within the matter or at any time we define properties or parameter as continuous function of space within the matters. So, it assume that each and every point in the matter there is a molecule (because all matter are composed of molecules) and hence, there is no discontinuity. This continuous and homogenous fluid media is called continuum. This is highly true for solid and liquid because molecule are highly pack but for gases if the pressure is very low, distance between the molecule increases cohesive force decreases and the concept of continuum may not be void.
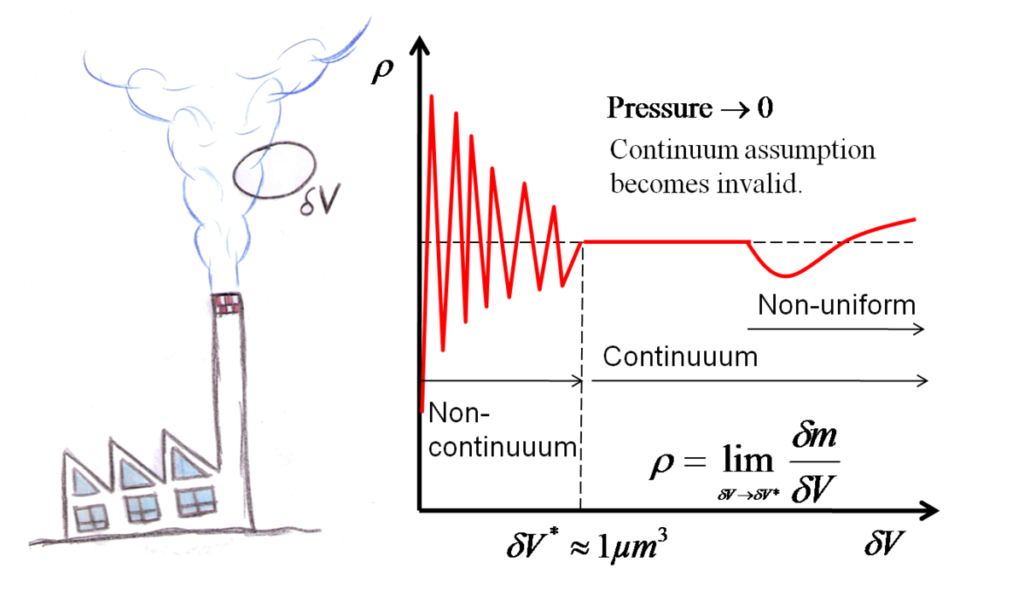
Source: https://en.wikiversity.org/wiki/File:Continuum_assumption_sketch.png
- Based on Knudsen number the validity of continuum concept is describe as if kn = λ /L < 0.01 then the concept of continuum is valid.
- Where,
- Distance between the molecule = λ
- Characteristics dimension of problem = L
Concept of control volume in fluid mechanics
Control volume is a volume in space of special interest for particular analysis. The surface of the control volume is referred as a control surface and is a closed surface. The surface is defined with relative to a coordinate system that may be fixed, moving or rotating. The control volume may be infinitesimally small or may be large and infinite size. The quality of matter and its identity in control volume may change with time but size and shape of control volume is fixed.
Example: Turbines, Compressors, Nozzle, Diffuser, Pumps etc.
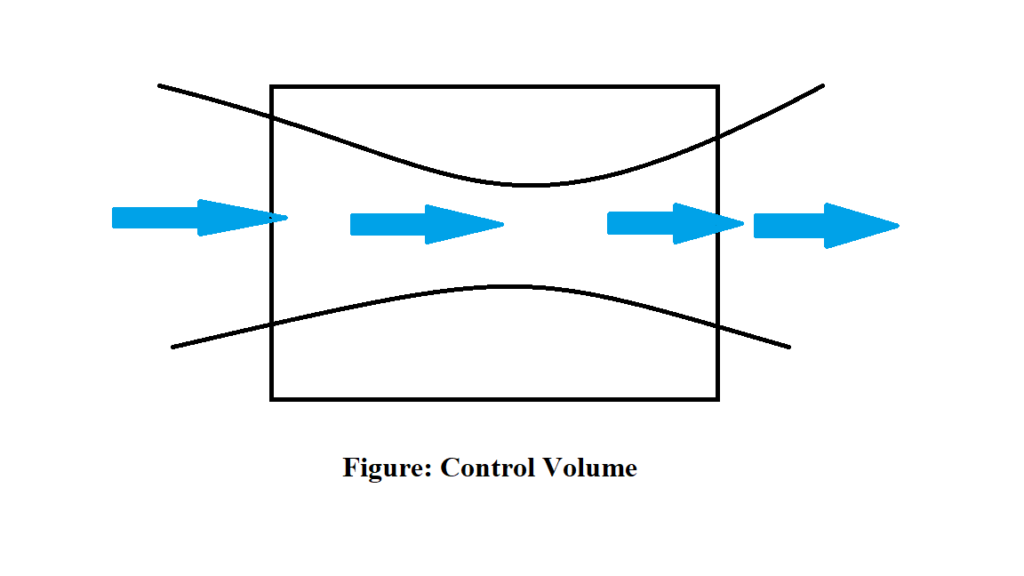
Vapour Pressure and Cavitation
Vapour Pressure
- Vapour pressure is the pressure exerted by the vapour on a liquid.
Consider a liquid which is restricted in a closed vessel. Assume that the temperature of liquid is 200 C and pressure is atmospheric. Then the liquid will vaporise at 1000 C . When it vaporise the molecule escape from the free surface of the liquid. These vapour molecules gets collected in the space between the free liquid surface and top of the vessel. The collection of vapours exerts a pressure on the liquid surface. This pressure is known as vapour pressure.

Source: https://www.hkdivedi.com/2017/12/vapour-pressure-and-cavitation.html
Cavitation
- Cavitation is a phenomenon when liquid passes from low pressure region to high pressure region.
- Consider a flowing liquid in a system and if the pressure at any point in this flowing liquid becomes equal to or less than the vapour pressure, the vaporization of the liquid starts. The bubbles of the vapour are carried by flowing liquid into the region of the high pressure where it collapse and give rise to high impact pressure.
- Due to the pressure developed by collapsing bubbles which so high that the material from the adjoining boundaries gets eroded and cavities are formed on them. This process is known as cavitation.

Newton’s Law of Viscosity
Newton’s Law of viscosity states that the shear stress in a flowing fluid is directly proportional to the rate of shear strain.
Mathematically,
Ʈ ∝(du)/(dy)
Ʈ = µ(du)/(dy)
Where,
Dynamic Viscosity = µ
Rate of shear stress = Ʈ
Velocity gradient = du/dy
Then,
µ= (Ʈ)/(du/dy)
Significance:
The fluid which follow Newton’s law of viscosity are called Newtonian fluids.
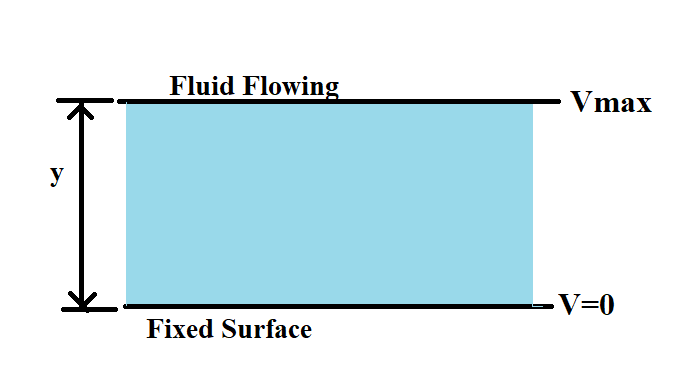
Consider two solid plates separated by a fluid body. Keeping lower plate fixed. The upper plate is applied with a force ‘F’ in x-direction which gives velocity ‘V’.
The experiment shows that:
- F α A where, A is surface area of plate in contact with liquid.
- F α V where, V is velocity.
- F α 1/y where, y is separation of two plates.
Now,
F α (AV)/y
Or, F = µ (AV)/y {where, µ is called coefficient of dynamic viscosity}
Or, (F)/A = µ(V)/y
Ʈ = µ (V)/y
Cohesion and Adhesion
Cohesion
Cohesion refers to the attraction of molecules for other molecules of the same kind and water molecule have strong cohesive force thanks to their ability to form hydrogen bond with one another. Also, cohesive forces are responsible for surface tension.
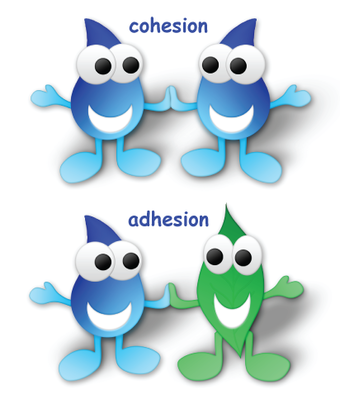
Adhesion
Adhesion is the attraction of molecule of one kind for molecules of a different kind, and it can be quite strong for water, especially with other molecules bearing positive or negative changes.
Surface tension
- Surface tension is defined as a tensile force acting on the surface of a liquid in contact with air (gas) or between two immiscible liquid.
- It is denoted by ‘σ’ and its unit is N/m .
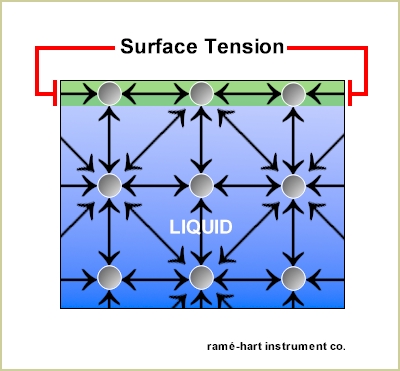
Source: http://www.ramehart.com/surface_tension.htm
Surface Tension on Liquid Droplet
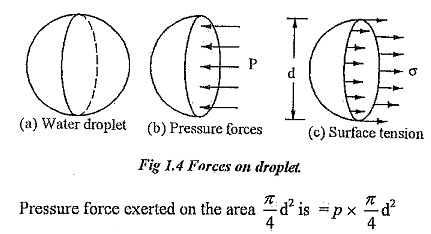
Source: https://www.eeeguide.com/surface-tension/
Tensile force = Surface tension * Circumference
Or, Tensile force = σ*π d
Pressure force = P * π/4 d2
At equilibrium,
σ*π* d = P * π/4 d2
Or, σ = (P * π/4 d2) / πd
Or, σ = Pd / 4
Surface Tension on a Hollow Bubble
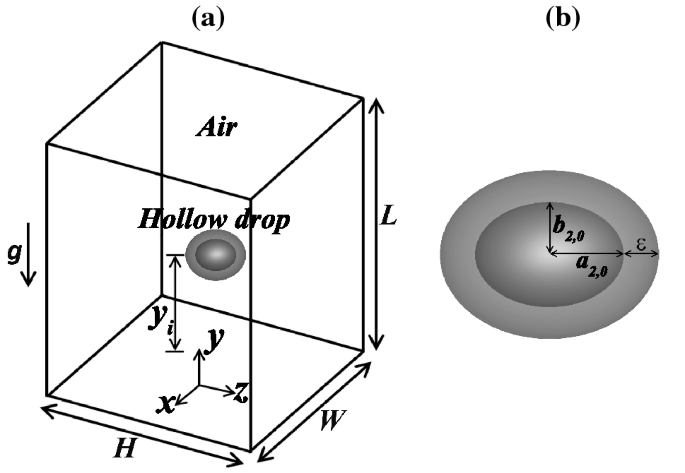
Source: https://link.springer.com/article/10.1007/s00162-020-00517-z
Tensile force = 2*(Surface tension * Circumference)
Or, Tensile force = 2* ( σ* πd)
Pressure force = P *π/4 * d2
At equilibrium,
2* ( σ* π d) = P * π/4 * d2
σ= Pd/8
Surface Tension on Liquid Jet
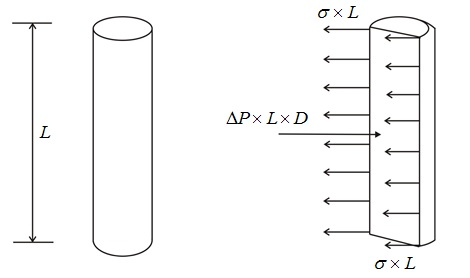
Tensile force = σ * 2L
Pressure force = P*L*d
At equilibrium,
σ* 2L= P*L*d
Or, σ = (P*L*d)/2L
σ = Pd/2
Capillarity or Meniscus effect
The phenomenon of rise or fall of a liquid in a small diameter tube is known a capillarity or meniscus effect.
Capillary Rise
It is only in wetting liquid i.e. whose adhesion force is greater. Example: Water.
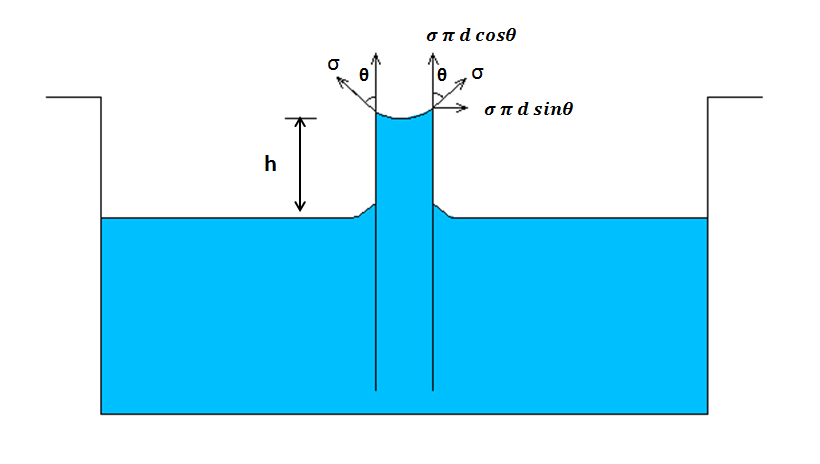
Source: https://socratic.org/questions/5a5349317c01493f36fa0910
Weight (W) = Mass (m)*Gravity (g)
Or, W = ρVg
Or, W= ρ*(A*h)*g
Where,
Density = ρ
Volume = V
Area = A
Height of rise = h
Tensile Force = Surface tension * Circumference * cosθ
Or, Tensile Force = σ*πd*cosθ
At equilibrium condition,
ρ *(A*h)*g = σ*πd*cosθ
Or, ρ *(πd2 /4 * h)*g = σ*πd*cosθ
Or, h = (4σ * cosθ)/ρgh
If Ɵ =0
Then,
h =4σ/ρgh
Capillary fall
It is only in non-wetting liquids i.e. inter-molecular force is greater.
Example: Mercury.
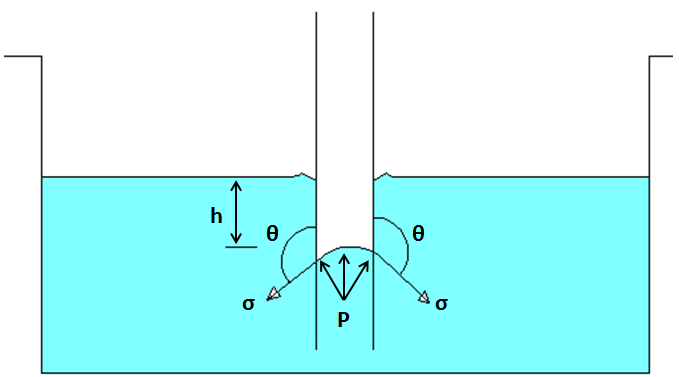
Source: https://www.hkdivedi.com/2017/12/capillarity-capillary-action-and.html
Hydrostatic force = Pressure * Area
Or, Hydrostatic force = ρgh*π/4 * d2
Tensile force = Surface tension * Circumference *
Or, Tensile force =σ * πd * cosθ
At equilibrium condition,
ρgh*π/4 * d2 = σ * πd * cosθ
Or, h =(σ * πd * cosθ)/ρg*π/4 * d2
h=(4σ * πd * cosθ)/ρg
Capillary rise or fall between two vertical parallel plates

Source: https://www.sciencedirect.com/science/article/abs/pii/S0021979715301661
Weight (W) = Mass (m) * Gravity (g)
Or, W= ρVg
Or, W= ρ (A*h) g
Or, W= ρ (L*t) h g
Tensile force= σ* 2L *cosθ
At equilibrium condition,
ρ (L*t) h g = σ* 2L*cosθ
Or, h =(σ* 2L*cosθ)/ρ (L*t) g
h =(2σcosθ)/ρtg
References: 1. A text book of fluid mechanics and hydraulic machines, Dr. RK Bansal, (2008), Laxmi publication(P) LTD.

